This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Opinion of the Slovak Academy of Sciences on vaccination

The opinion is based on scientific facts and medical evidence derived from reliable and publicly available sources. It was prepared by the leading scientists of the Institute of Virology of the Biomedical Research Center SAS, and represents the official opinion of the SAS with the supportive opinion of the members of the Presidium of SAS.
We consider vaccination to be the most effective and least risky way of preventing infectious diseases.
- Vaccination averts two to three million deaths worldwide per year.
- The direct result of vaccination is the disappearance of smallpox (Variola) and the almost complete elimination of polio.
- Between 2000 and 2018, vaccination resulted in a significant reduction in measles deaths with an estimated saving of more than 23 million lives.
- Thanks to vaccination, there are no more life-threatening infectious diseases in Slovakia. Until the first year of life, children are administered three doses of vaccines against seven viral or bacterial diseases, and up to the 13th year of life, vaccines against three other diseases are administered. Vaccination coverage is 96 to 98%, which allows the maintenance of collective immunity and protection of children who cannot be vaccinated due to health contraindications.
Vaccination is a universal, scientifically proven mechanism for “training” the immune system to recognise the infection and prevent disease.
- Vaccination is based on the body’s ability to recognise an element that is foreign to the body (the so-called antigen) and to develop a defence immune response against it.
- The vaccine mimics and “trains” this foreign element to be prepared for a real infection, to respond to it quickly and effectively, to prevent the virus from multiplying in the body, and to prevent disease.
- The prevention or alleviation of the disease is ensured, on the one hand, by vaccine-induced antibodies, which bind to the virus particles and prevent the virus from entering the cells, and, on the other hand, by activating the immune cells that are needed for antibody production, destruction of virus-infected cells and for the development of immune memory.
- Vaccines may contain an ingredient that increases their ability to induce immunity (the so-called adjuvants).
- Vaccination may cause temporary side effects (redness and pain at the injection site, fever, headache, muscle and joint pain) which are a manifestation of the activation of the immune system needed to make an immune response.
- About 1 to 10 people in 1 million vaccinated may have an acute allergic reaction to any of the ingredients of the vaccines. These reactions can be effectively suppressed in the outpatient clinic immediately after vaccination.
- In the case of some vaccines, autoimmune reactions that have occurred within one to two months after vaccination have been recorded rarely in individuals who are genetically predisposed to immune disorders. However, similar autoimmune reactions can be induced in these individuals to an even greater extent by the infectious viruses themselves. Current scientific knowledge shows that their cause is the structural similarity of parts of some viral proteins with proteins of the human body, the so-called molecular mimicry. Therefore, increased attention is paid to the safety and contraindications of vaccines.
- The implementation of the vaccine is always preceded by a thorough clinical trial on tens of thousands of volunteers and a thorough analysis of the effectiveness and, in particular, the safety of the vaccine by independent national and transnational institutions.
Several types of vaccines can provide reliable and safe protection against infectious diseases.
- Live attenuated vaccines are used e.g. against measles, mumps, and rubella in the MMR triple vaccine. They are made up of viruses which ability to cause the disease has been significantly weakened by the long-term cultivation in the laboratory.
- Inanimate virus vaccines are used e.g. against polio. They are inactivated by chemical or radiation treatment and usually contain adjuvants to promote efficacy.
- Subunit vaccines consist of viral proteins or their fragments that represent dominant viral antigens. An adjuvant is added to increase their efficacy. Their subgroup is non-infectious, virus-like particles formed by a single viral protein that is able to assemble into an empty virus-mimicking shell. They are used e.g. against hepatitis B.
- Vector vaccines are based on non-pathogenic viruses (e.g. adenoviruses) that have removed regions necessary for reproduction. Instead, they have the inserted gene for the antigen of the virus to which it is desired to elicit immunity. They are highly effective and have a good safety profile. This type of vaccine is used e.g. against Ebola.
- Nucleic acid vaccines consist of a fragment of DNA or RNA that encodes a viral antigen or its part. Current technologies focus, in particular, on mRNA-based vaccines, which contain modifications to increase the stability and efficacy of antigen production. This type of vaccine has been in development for several years, undergoing the first phase of clinical trials for Zika and HIV viruses.
Effective and safe vaccines against COVID-19 are/will be available in Slovakia.
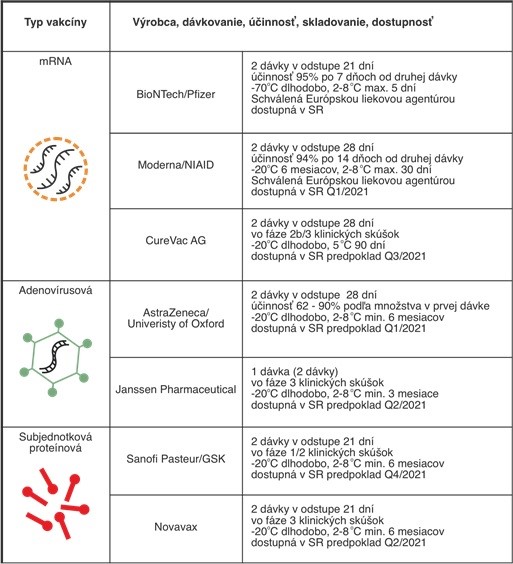
- An mRNA-based vaccine encoding the S protein of the SARS-CoV-2 virus (Pfizer/BioNTech) is currently used in Slovakia. The mRNA is packaged in a lipid nanoparticle, which increases its stability and improves efficacy. Clinical trials have proved a good safety profile and efficacy of 52 % after 12 days since the first dose and 95 % after seven days since the second dose. The European Medicines Agency (EMA) has already approved an mRNA vaccine from the Moderna company.
- Both the EMA and the vaccine based on the replication-defective chimpanzee adenovirus producing the S protein SARS-CoV-2 (University of Oxford/Astra-Zeneca) should be granted permission soon. An advantage of using chimpanzee adenovirus as a vaccine base is the reduction of non-specific immunity caused by the existence of antibodies to human adenoviruses that are common in the population. This results in a higher specific immune response against the coronavirus antigen. Clinical trials have proved vaccine safety and efficacy of 62 to 90% after the second dose, depending on the amount.
- Subunit protein vaccines are also at a high stage of development, the characteristics of which are given in the attached table.
- Some aspects of COVID-19 vaccines need to be further monitored. These include the persistence of antibody and memory cellular immunity to SARS-CoV-2 virus after vaccination and the contribution of non-specific immunity to common coronaviruses (causing colds) to the immune response to vaccination (as well as to the infection itself).
Vaccination against COVID-19 is of great benefit to the individual and society as a whole.
- It protects the vaccinated person from the onset and/or severe course of the disease, resulting in the direct saving of human lives.
- By preventing the occurrence of COVID-19, the vaccine will also protect against the adverse health effects that may persist for a long time after overcoming SARS-CoV-2 infection, thereby improving the quality of life of the vaccinated person.
- The vaccine-induced decrease in morbidity will relieve the burden on healthcare and allow the resumption of treatment of patients with other diseases that have been postponed or discontinued due to the pandemic. This will result in an indirect saving of human lives.
- After reaching a certain percentage of vaccinated people in the population (usually reported to be more than 70 %), it will produce collective immunity limiting the spread of the virus and preventing infection of those people who cannot be vaccinated due to health reasons. This will lead to an indirect saving of their lives.
- Vaccination is the only effective and ethically acceptable way out of the pandemic that will open a return to normal life and allow us to revitalise social and economic activities, including education.
The Slovak Academy of Sciences considers vaccination against COVID-19 to be meaningful and socially necessary. It recommends it to all the inhabitants of Slovakia whose health condition so permits and who care about protecting themselves and others from the dangerous disease COVID-19.
Information sources:
- https://data.unicef.org/topic/child-health/immunization/
- https://www.uvzsr.sk/docs/info/ockovanie/Ockovaci_kalendar_pre_pravidelne_povinne_ockovanie_deti_a_dospelych_na_rok_2021.pdf
- Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cellular & Molecular Immunology 2018 March 5; 15: 586-594; doi:10.1038/cmi.2017.151
- https://www.mayoclinicproceedings.org/article/S0025-6196(20)30827-2/fulltext
- Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013 Dec;13(6):421-33. doi: 10.2174/1566523213666131125095046. PMID: 24279313; PMCID:PMC4507798.
- Jeyanathan, M., Afkhami, S., Smaill, F. et al.Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 20, 615–632 (2020). https://doi.org/10.1038/s41577-020-00434-6
- Ramasamy et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet 2020. Dec 19; 396: 1980-1993.
- Pardi et al, Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses J Exp Med4 June 2018; 215 (6): 1571–1588 https://doi.org/10.1084/jem.20171450
- Alberer et al, Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. 390:1511–1520.https://doi.org/10.1016/S0140-6736(17)31665-3
- Walsh et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med 2020;383:2439-50. DOI: 10.1056/NEJMoa2027906
- Polack et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; DOI: 10.1056/NEJMoa2034577
Tab. Characteristics of vaccines that are/will be available in Slovakia (in Slovak language)
PRESS RELEASE (in the Slovak language)
Photo: Katarína Gáliková
Translated by: SAS







